The goal of SIMCor is to demonstrate how the use of in-silico testing methodologies, including virtual patient cohorts and modelling and simulation (M&S), can support the verification, validation and regulatory approval of new and orphan medical devices. Most importantly, the project aims to show how M&S can reduce the use of animal testing and the size and duration of clinical trials, helping to adjust device design beforehand and deliver safer and more effective devices, in a shorter time and at lower costs for healthcare systems.
The project, now in its last 6 months of implementation, has succeeded in developing and validating a Virtual Cohort Generator (VCG), now embedded in its Virtual Research Environment (VRE), able to generate cohorts of aortic stenosis and heart failure patients, as well as healthy pigs, for conducting in-silico testing of TAVI and PAPS devices.
Now, leveraging these resources, the project is going through its final step, which consists of the conduction of 4 in-silico clinical trials (ISCTs) to evaluate specific questions of interest for TAVI and PAPS, to validate the overall reliability and applicability of its approach. This effort will be carried out through the collaboration of IIB, TUE, and CHA, carrying out the ISCT simulations for TAVI and PAPS, ECRIN, which supports the study design and statistical analysis, TUE and UTBV, which developed the VCG and its implementation within the VRE. The ISCT studies will also serve the purpose of validating the usability of the R-Statistical Environment, a statistical analysis environment developed by ECRIN in the R programming language for the specific purpose of ISCT design, conduction and result analysis.
For the ISCTs, from an initial set of candidate application scenarios, 4 were selected for analysis:
- TAVI-1 – Effects of convergent and divergent left ventricular outflow tract (LVOT) shapes on paravalvular leakage (PVL): use of computational model predictions to assess the effect of the LVOT shape on clinical outcomes such as PVL.
- TAVI-3 – Effects of calcification on PVL after virtual TAVI: use of computational model predictions to estimate the impact of annulus calcification severity on clinical outcomes of TAVI.
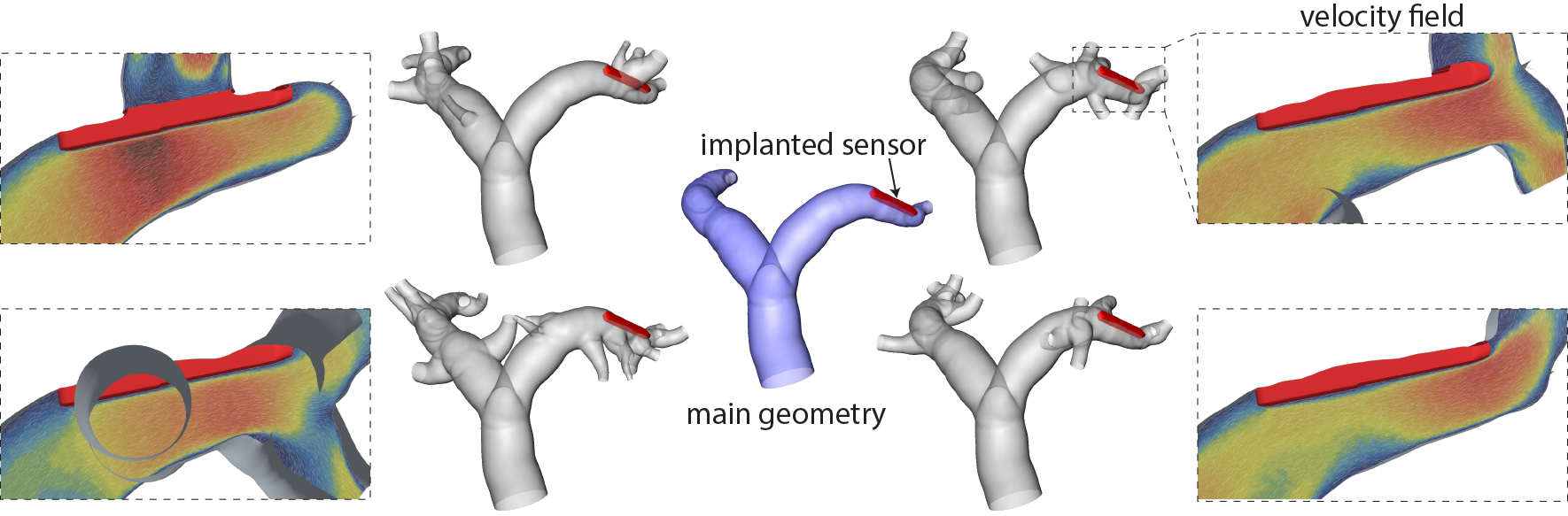
- PAPS-1 – Effects of side branches: use of virtual cohorts of porcine pulmonary arteries to assess the impact of side branches on device implantation and intravascular haemodynamics and therefore the risk for device thrombosis. Different side branch configurations are generated for the same main vessel geometries, allowing to analyse these effects independent from other variations.
- PAPS-4 – Translation towards human application: calculation and comparison of hemodynamic and structural mechanical parameters between the chronic animal experiment and human application, based on finite element analysis, for assessing device migration and perforation and computational fluid dynamics for assessing device thrombosis.
For each scenario, a standardised template was used to describe key assessment criteria, including Context of Use, Question of Interest, engineering outputs, targeted clinical outcomes, impact on clinical trials and medical device design). Another standardised template was developed to specify the essential protocol elements to be applied to the four scenarios.
The detailed description, protocols and results of all ISCTs will be reported in Deliverable 10.2 – Impact analysis on clinical and preclinical trials, which will be delivered by ECRIN by June 2024 and made publicly available in Zenodo after the approval of the European Commission’s appointed reviewers.